FDA issues emergency-use authorization for remdesivir to treat hospitalized patients with severe Covid-19 (cnn.com)
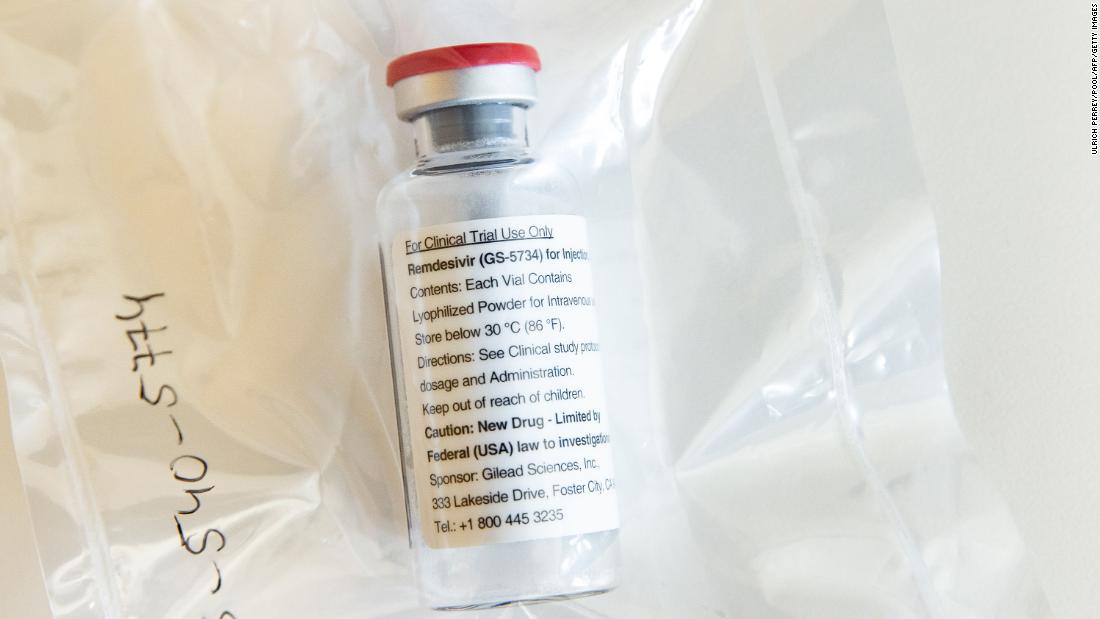
The experimental drug remdesivir has been approved to treat hospitalized patients with severe Covid-19, the US Food and Drug Administration said in a letter on Friday.
Remdesivir is the first authorized therapy drug for Covid-19 in the United States, FDA Commissioner Stephen Hahn said on Friday.
"This is an important clinical advance that showed a statistically significant reduction in time to recovery for patients with Covid-19 and is the first authorized therapy for Covid-19." Hahn said.
- Log in to post comments