
Moderna Inc.’s vaccine candidate against Covid-19 protected against the virus in a trial that inoculated 16 monkeys, an encouraging step on the path to a defense for humans against the pandemic.

Moderna Inc.’s vaccine candidate against Covid-19 protected against the virus in a trial that inoculated 16 monkeys, an encouraging step on the path to a defense for humans against the pandemic.

The risk of nasty side effects in the Moderna and Oxford trials should be made clear now, before it ends up as fodder for the skeptics.
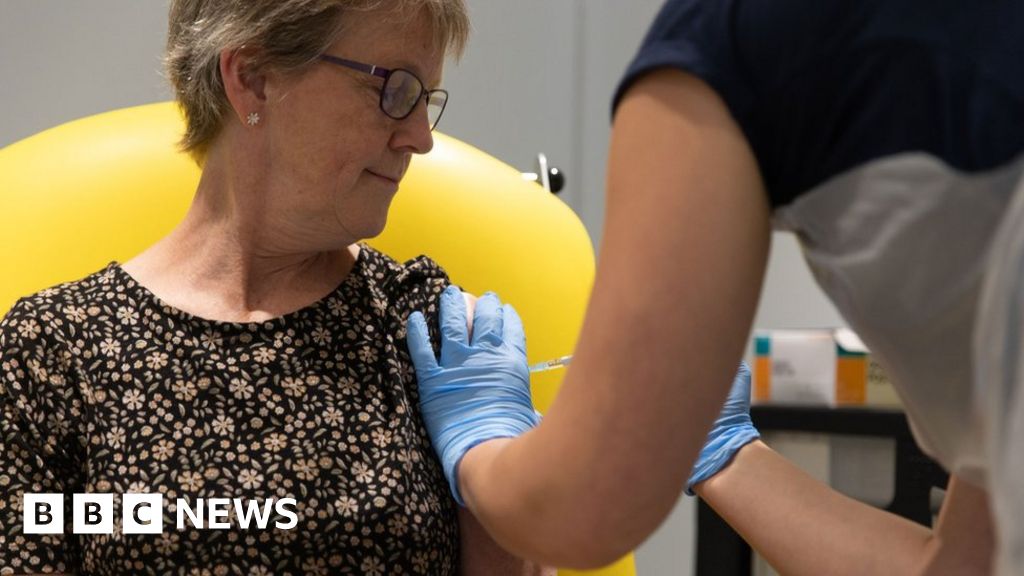
A coronavirus vaccine developed by the University of Oxford appears safe and triggers an immune response.
Trials involving 1,077 people showed the injection led to them making antibodies and T-cells that can fight coronavirus.
The findings are hugely promising, but it is still too soon to know if this is enough to offer protection and larger trials are under way.


Teachers in Florida are suing the state to block an emergency order requiring schools to open next month with in-person instruction. They say, with the surge of coronavirus cases, the order violates a provision in Florida's constitution requiring the state to ensure schools are operated safely.
The United States has secured almost a third of the first one billion doses planned for AstraZeneca’s experimental COVID-19 vaccine by pledging up to $1.2 billion, as world powers scramble for medicines to get their economies back to work.
The vaccine, previously known as ChAdOx1 nCoV-19 and now as AZD1222, was developed by the University of Oxford and licensed to AstraZeneca. Immunity to the new coronavirus is uncertain and so the use of vaccines is unclear.
The United States will pump up to $1.2 billion into developing AstraZeneca’s potential COVID-19 vaccine and said on Thursday it would order 300 million doses, as the White House seeks solutions to the coronavirus pandemic.
The commitment provides for a possible U.S.-based clinical trial this summer involving 30,000 volunteers and adds fuel to the British drugmaker’s efforts to develop a vaccine for the disease, one of around 100 which are under way worldwide.

Controversial trials in which volunteers are intentionally infected with Covid-19 could accelerate vaccine development, according to the World Health Organization, which has released new guidance on how the approach could be ethically justified despite the potential dangers for participants.
The first U.S. patients have been dosed in a clinical trial testing whether four experimental messenger RNA (mRNA) vaccine candidates can prevent infection with the virus that causes 2019 coronavirus disease (COVID-19).
The trials are being conducted at the NYU Grossman School of Medicine and the University of Maryland School of Medicine, the drugmaker said Tuesday.

Scientists at the Los Alamos National Laboratory published a study on Thursday that indicates the now-prevalent strain of coronavirus is a more contagious version of the pathogen first reported in Wuhan, China.