FDA issues emergency use authorization for Pfizer/BioNTech Covid-19 vaccine (cnn.com)
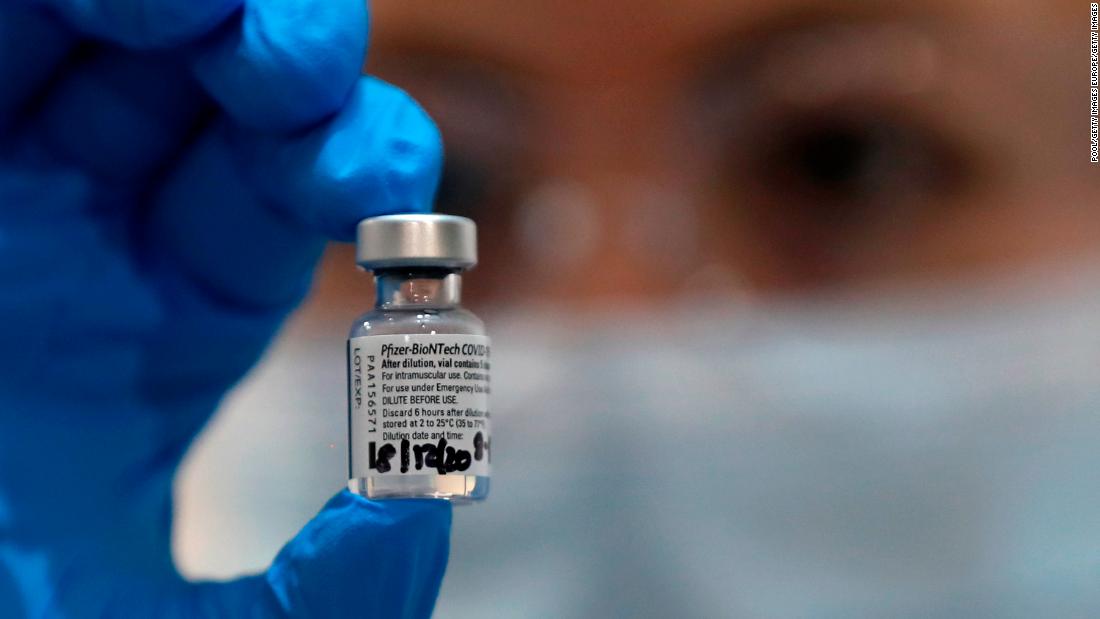
(CNN)The US Food and Drug Administration has authorized the first Covid-19 vaccine for emergency use in the United States. Millions of doses of the Pfizer and BioNTech coronavirus vaccine, which has been found to be 95% effective, will be soon shipped around the country so vaccinations can begin within days.
An emergency use authorization means what its name suggests: a medical product gets special authorization by the FDA to be used during an emergency -- but it is short of a full approval. Pfizer would have to file a separate application for its vaccine to be fully licensed by the FDA.
- Log in to post comments