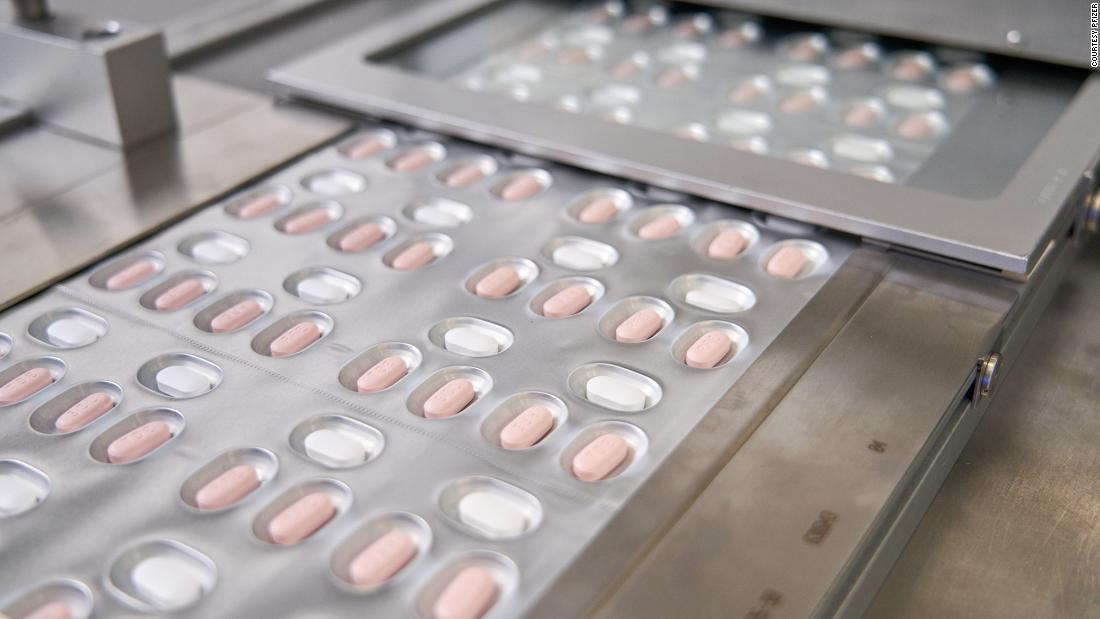
The US Food and Drug Administration on Thursday authorized Merck's antiviral pill, molnupiravir, to treat Covid-19 "for the treatment of mild-to-moderate coronavirus disease (COVID-19) in adults with positive results of direct SARS-CoV-2 viral testing, and who are at high risk for progression to severe COVID-19, including hospitalization or death, and for whom alternative COVID-19 treatment options auth




/cloudfront-us-east-2.images.arcpublishing.com/reuters/4KWTOARBKJONRCSVC7LSKRPUR4.jpg)