
Countries scrambling to prevent fresh Covid-19 outbreaks driven by the Omicron variant have been offered hope by a UK study that found booster jabs “massively” strengthen the body’s defences against the coronavirus.

Countries scrambling to prevent fresh Covid-19 outbreaks driven by the Omicron variant have been offered hope by a UK study that found booster jabs “massively” strengthen the body’s defences against the coronavirus.
/cloudfront-us-east-2.images.arcpublishing.com/reuters/KR26TR5HDVKFTCJPRUQFPPYIVI.jpg)
NEW YORK, Dec 3 (Reuters) - The Omicron variant of the virus that causes COVID-19 likely acquired at least one of its mutations by picking up a snippet of genetic material from another virus - possibly one that causes the common cold - present in the same infected cells, according to researchers.
This genetic sequence does not appear in any earlier versions of the coronavirus, called SARS-CoV-2, but is ubiquitous in many other viruses including those that cause the common cold, and also in the human genome, researchers said.

MANILA, Philippines (AP) — Measures used to counter the delta variant should remain the foundation for fighting the coronavirus pandemic, even in the face of the new omicron version of the virus, World Health Organization officials said Friday, while acknowledging that the travel restrictions imposed by some countries may buy time.
/cloudfront-us-east-2.images.arcpublishing.com/reuters/QZ7KYBJJLRNNJFXE52XQ3FTUYU.jpg)
WASHINGTON/BERLIN, Dec 2 (Reuters) - President Joe Biden on Thursday laid out his strategy to fight th

Johannesburg, South Africa (CNN)In the early days of November, laboratory technicians at Lancet Laboratories in Pretoria, South Africa, found unusual features in samples they were testing for the coronavirus.

The CDC said Wednesday it has confirmed the first U.S. case of the new, heavily mutated coronavirus variant called omicron, which was detected in Northern California.
White House chief medical advisor Dr. Anthony Fauci said the patient, who was fully vaccinated, had just returned to the San Francisco area Nov. 22 after traveling in South Africa and tested positive Nov. 29.

The World Health Organization said Wednesday that 23 countries across the world have reported cases of the highly mutated omicron Covid-19 variant.
“At least 23 countries from five of six WHO regions have now reported cases of omicron and we expect that number to grow,” WHO Director-General Tedros Adhanom Ghebreyesus told reporters during an update Wednesday in Geneva.
/cloudfront-us-east-2.images.arcpublishing.com/reuters/RMUP4WTMCVKSDEOVAWRH7HG24E.jpg)
SYDNEY, Nov 30 (Reuters) - The head of drugmaker Moderna (MRNA.O) said COVID-19 vaccines are unlikely to be as effective against the Omicron variant of the coronavirus as they have been previously,
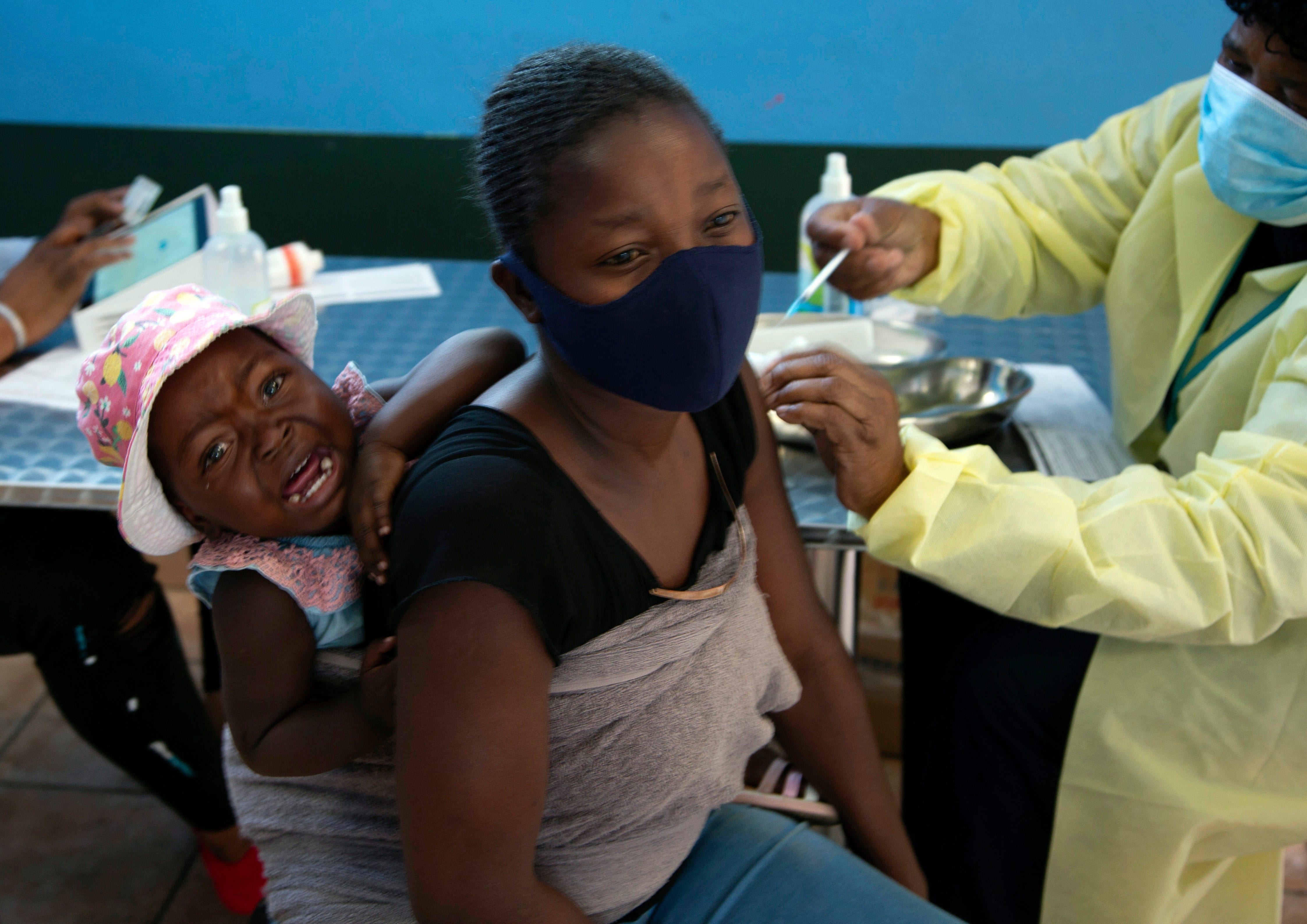
A COVID-19 variant spreading in South Africa was dubbed "omicron" and classified a "variant of concern" by the World Health Organization on Friday, as the U.S. and other nations reacted to the newly discovered variant with travel restrictions.
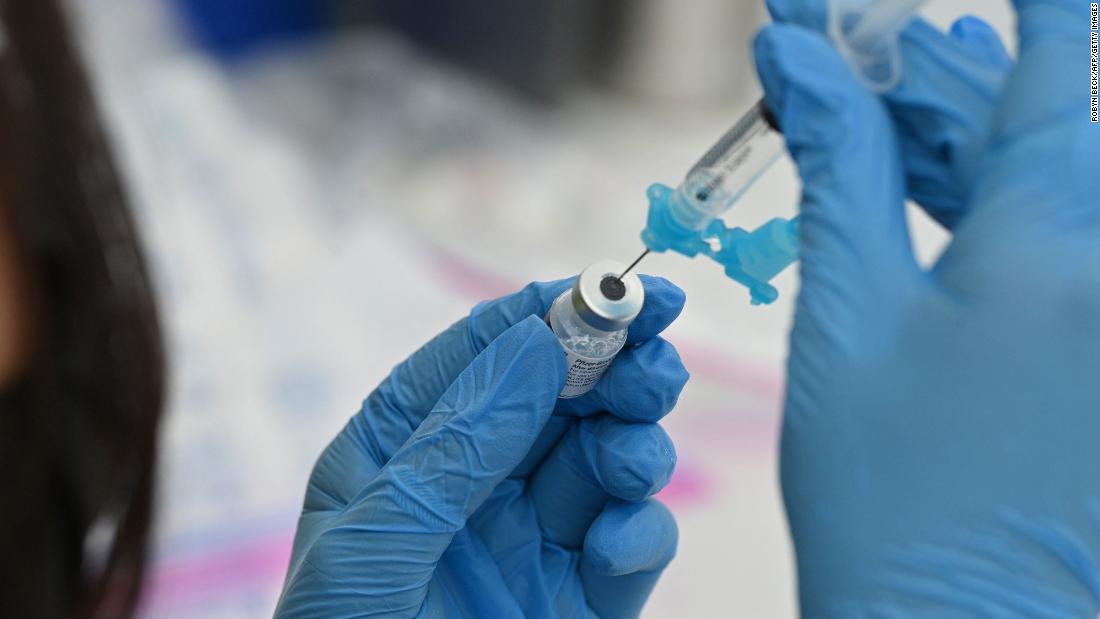
US Centers for Disease Control and Prevention Director Dr. Rochelle Walensky endorsed the use of Covid-19 vaccine boosters Friday for all adults.